New rules on the use and labelling of products containing titanium dioxide come into effect on 1st October this year.
The upcoming change in EU regulations concerns using Titanium Dioxide in mixtures, which may well affect local remanufacturers and re-fillers of toner cartridges.
Titanium Dioxide is utilised by many industries, primarily as a white pigment. It is included in some toner formulations as a surface additive to control how a toner charges and help regulate its flowability.
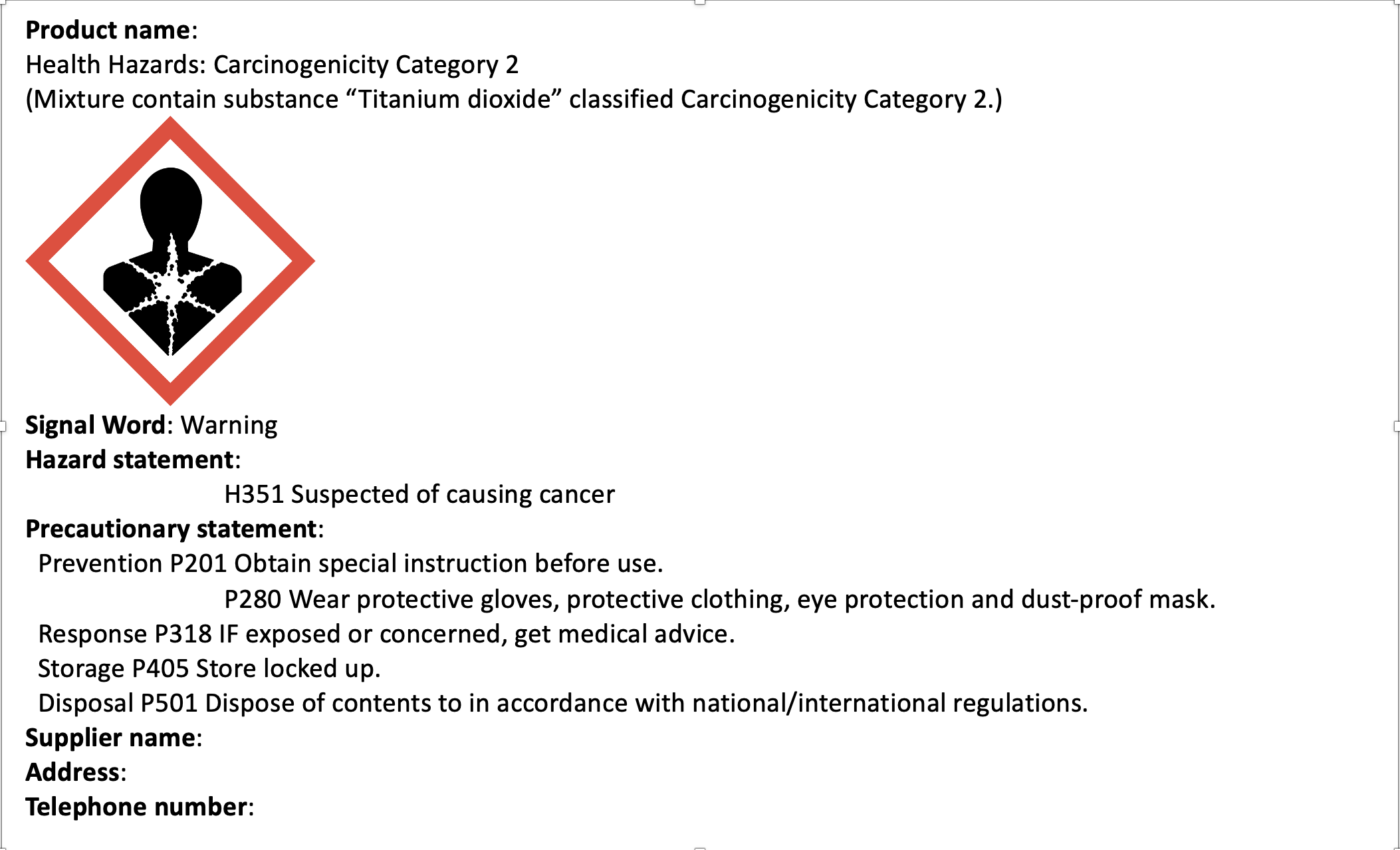
In February 2020, the European Chemical Agency (ECHA) published the delegated regulation classifying titanium dioxide (TiO2) as a suspected carcinogen (Category 2) via inhalation under its EU Regulation No 1272/2008 on classification, labelling and packing (CLP) of substances and mixtures.
This regulation comes into effect on 1 October 2021 when the CLP regulations require hazard labels on products containing hazardous substances or mixtures when put on the market to provide information to the consumer.
Important: Cartridges that contain toner powders with 1% or more titanium dioxide content must be labelled with a specific warning pictogram on the package as indicated in the following figure.
Major hardware manufacturers such as Canon, Fujifilm, Ricoh, Konica-Minolta and Kyocera have responded to this regulation change and made changes necessary to become compliant.
Peter Knak, General Manager, IMEX Europe, told The Recycler: “IMEX started work on improving formulations with more than 1% TiO2 content well before the deadline and have dedicated substantial laboratory time to ensure that the product quality is maintained throughout any formulation adjustments necessary to make these toners compliant. Where necessary the TiO2 level has been adjusted so that our customers will not have to introduce hazard labelling on finished products, which could have an adverse impact their sales.”
From September 2021, Blue Angel Mark (BAM) will not certify the Remanufactured Toner Module (DE-UZ 171) if a toner contains more than 1% titanium dioxide. We will meet this requirement throughout our product range.
Not everyone agrees: The Titanium Dioxide Manufacturers Association (TDMA) say on their website: “There is no scientific evidence of cancer in humans from exposure to titanium dioxide. The classification is based on one rat inhalation study which was conducted in excessive overload conditions. The TDMA believe this study is not an acceptable scientific basis for the classification and a thorough weight of evidence assessment confirms that TiO2 does not induce cancer and does not have an intrinsic property to cause cancer.
The EU authorities have underlined in the classification that the suspected hazard could occur if dust is inhaled in extremely high concentrations over a long period of time.”
Editor: We would like to thank Peter Knak and the IMEX research team for the detailed background information and help to produce this industry alert.